Google’s AlphaFold 3 AI system takes on the mystery of molecules
The fight against diseases has been a constant pursuit in the medical field. From the dawn of medicine, researchers have tirelessly strived to understand the intricate workings of the human body and the microscopic foes that threaten our health. One crucial area of focus has been on medications, those life-saving molecules designed to interact with our biology and combat illnesses. However, efficiently designing these drugs has long been a challenging process, often requiring years of research and testing.
This is where a new tool emerges, armed with the power of artificial intelligence (AI). Google DeepMind, the company’s AI research lab, has introduced AlphaFold 3, a revolutionary molecular prediction model.
So, what exactly is AlphaFold 3 and how does it propose to change the landscape of drug discovery?
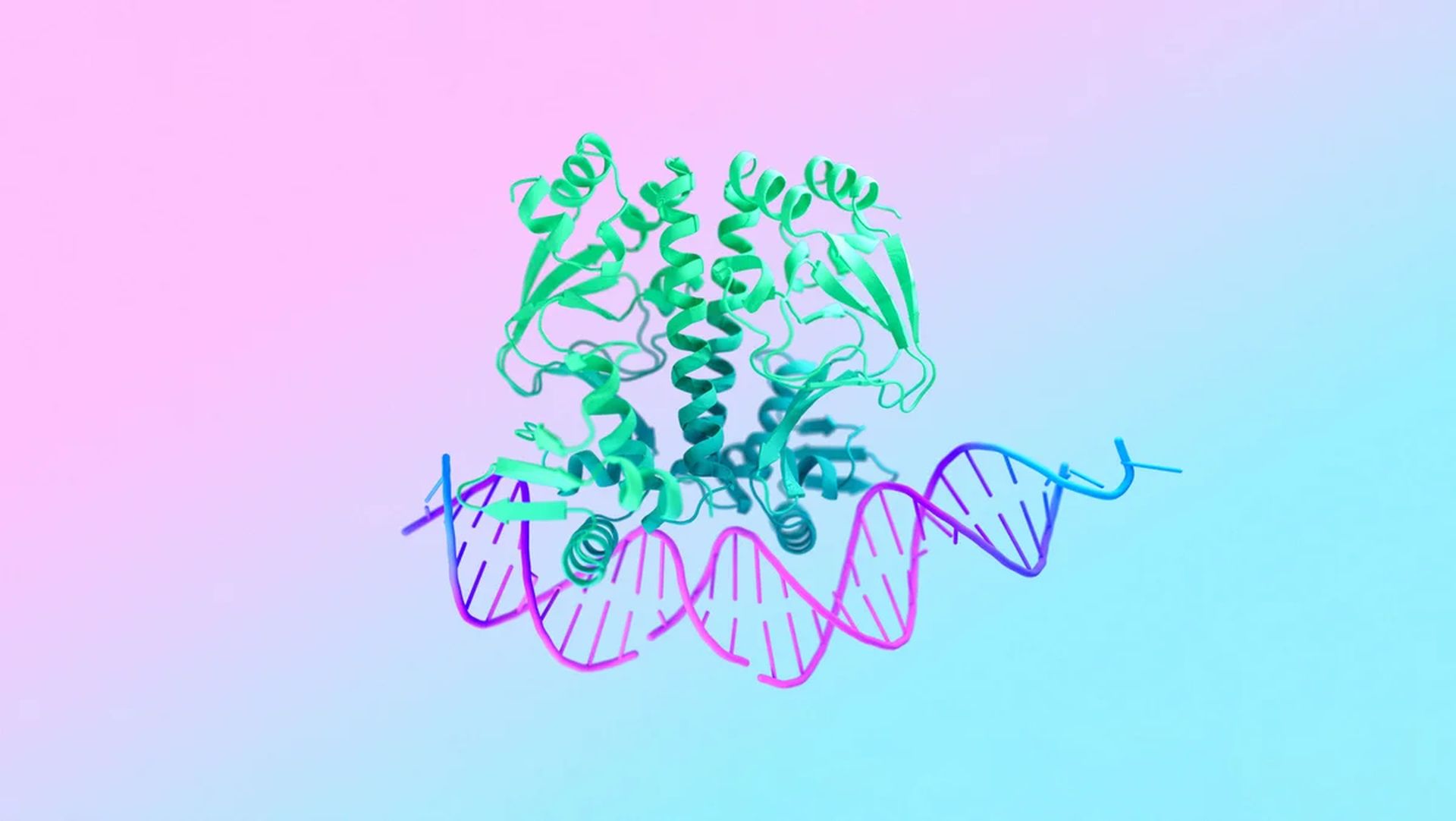
AlphaFold 3 observes the dance of molecules in living cellsImagine billions of tiny machines working together inside every cell of your body. These machines, built from proteins, DNA, and other molecules, orchestrate the complex processes of life. But to truly understand how life works, we need to see how these molecules interact with each other in countless combinations.
In a recent paper by Google, researchers describe how AlphaFold 3 can predict the structure and interactions of all these life molecules with unmatched accuracy. The model significantly improves upon previous methods, particularly in predicting how proteins interact with other molecule types.
AlphaFold 3 builds on the success of its predecessor, AlphaFold 2, which made a breakthrough in protein structure prediction in 2020. While AlphaFold 2 focused on proteins, AlphaFold 3 takes a broader view. It can model a wide range of biomolecules, including DNA, RNA, and small molecules like drugs. This allows scientists to see how these different molecules fit together and interact within a cell.
The model’s capabilities stem from its next-generation architecture and training on a massive dataset encompassing all life’s molecules. At its core lies an improved version of the Evoformer module, the deep learning engine that powered AlphaFold 2. AlphaFold 3 then uses a diffusion network to assemble its predictions, similar to those used in AI image generation. This process starts with a scattered cloud of atoms and gradually refines it into a precise molecular structure.
The model’s ability to predict molecular interactions surpasses existing systems. By analyzing entire molecular complexes as a whole, AlphaFold 3 offers a unique way to unify scientific insights into cellular processes.
How does AlphaFold 3 work?AlphaFold 3’s ability to predict the structure and interactions of biomolecules lies in its sophisticated architecture and training process. Here’s a breakdown of the technical details:
1. Deep learning architecture: The foundationAlphaFold 3 relies on a sophisticated deep learning architecture, likely an enhanced version of the Evoformer module used in its predecessor, AlphaFold 2. Deep learning architectures are powerful tools capable of identifying complex patterns within data. In AlphaFold 3’s case, the patterns of interest lie within the amino acid sequences of biomolecules.
2. Processing the blueprint: Input and attention mechanismsThe model likely receives the amino acid sequence of a biomolecule as input. It then employs attention mechanisms to analyze the sequence and identify critical relationships between different amino acids. Attention mechanisms allow the model to focus on specific parts of the sequence that are most relevant for predicting the final structure.
3. Building the molecule: Diffusion networks take overAfter processing the input sequence, AlphaFold 3 utilizes a diffusion network to assemble its predictions. Diffusion networks are a type of generative model that progressively refine an initial guess towards a more accurate output. In this context, the initial guess might be a scattered cloud of atoms representing the potential locations of each atom in the biomolecule.
Xaira secures a billion-dollar bet on the future of AI drug discovery
Through a series of steps, the diffusion network iteratively adjusts these positions, guided by the information extracted from the sequence and inherent physical and chemical constraints.
4. Obeying the laws of nature: Physical and chemical constraintsAlphaFold 3 likely incorporates knowledge of physical and chemical constraints during structure prediction. These constraints ensure the predicted structures are realistic and adhere to scientific principles. Examples of such constraints include bond lengths, bond angles, and steric clashes (atoms being too close together).
5. Learning from examples: Training on vast datasetsAlphaFold 3’s impressive accuracy is attributed to its training on a massive dataset of biomolecules. This data likely includes known protein structures determined experimentally using techniques like X-ray crystallography. By analyzing these known structures alongside their corresponding amino acid sequences, AlphaFold 3 learns the intricate relationship between sequence and structure, enabling it to make accurate predictions for unseen biomolecules.
Applications in drug discovery are vastOne of the most exciting applications of AlphaFold 3 lies in drug design. The model can predict how drugs interact with proteins, offering valuable insights into how they might influence human health and disease.
For example, AlphaFold 3 can predict how antibodies bind to specific proteins, a crucial aspect of the immune response and the development of new antibody-based therapies.
Isomorphic Labs, a company specializing in AI-powered drug discovery, is already collaborating with pharmaceutical companies to utilize AlphaFold 3 for real-world drug design challenges. The goal is to develop new life-saving treatments by using AlphaFold 3 to understand new disease targets and refine existing drug development strategies.
 Application studies for AlphaFold 3 in drug discovery have begun (Image credit)
Making the power accessible
Application studies for AlphaFold 3 in drug discovery have begun (Image credit)
Making the power accessible
To make AlphaFold 3’s capabilities available to a wider scientific community, Google DeepMind launched AlphaFold Server, a free and user-friendly research tool. This platform allows scientists worldwide to harness the power of AlphaFold 3 for non-commercial research. With just a few clicks, biologists can generate structural models of proteins, DNA, RNA, and other molecules.
AlphaFold Server empowers researchers to formulate new hypotheses and accelerate their work. The platform provides easy access to predictions regardless of a researcher’s computational resources or machine learning expertise. This eliminates the need for expensive and time-consuming experimental methods of protein structure determination.
Sharing responsibly and looking aheadWith each iteration of AlphaFold, Google DeepMind prioritizes responsible development and use of the technology. They collaborate extensively with researchers and safety experts to assess potential risks and ensure the benefits reach the broader scientific community.
AlphaFold Server reflects this commitment by providing free access to a vast database of protein structures and educational resources. Additionally, Google DeepMind is working with partners to equip scientists, particularly in developing regions, with the tools and knowledge to leverage AlphaFold 3 for impactful research.
AlphaFold 3 offers a high-definition view of the biological world, allowing scientists to observe cellular systems in their intricate complexity. This newfound understanding of how molecules interact promises to revolutionize our understanding of biology, pave the way for faster drug discovery, and ultimately lead to advancements in human health and well-being.
Featured image credit: Google
